Enhancement program of the Kuda Huraa house
reef
Enhancement program of the Kuda Huraa house reef
Hypothetical processes operating at the site
Hypothetical processes operating at the site
Hypothetical processes operating at the site
Impacts of recreational diving
Activities of the project (will include the scientific protocol)
Introduction
The Kuda Huraa
house reef is located on the lee side of the large reef system of Huraa in
North Male Atoll. The most important feature on the lee side is a fracture in
the reef structure south west of Kuda Huraa. This break usually referred to as
the Kuda Hura House Reef, is a preferred dive site for the diving school,
especially for beginners and for the orientation dives and thus the project
will be carried out at this site. Diving is an important income earner for the
tourism industry in the Maldives, and this industry could be affected by a
degradation of the bio-physical status of the reefs.
At the site, possible causes of reef degradation include:
- coral
bleaching, when corals stressed either by an increase in temperature,
salinity, light, sedimentation, aerial exposure and pollutants lose or
expel their symbiotic zooxanthellae (Glynn, 1993)
- impacts
from diving, when an inexperienced diver breaks coral with their fins
- algal
overgrowth, algae and corals competing for space (Hughes, 1994)
- increased nutrient levels due to human
presence on the island
- various
outbreaks of predators such as the crown of thorns starfish.
Even though anthropogenic activities are partly responsible for reef
degradation, the single most important event shaping the reef landscape at
present is the 1998 coral bleaching event. Of unprecedented reported scale, it
decimated the coral colonies (reference needed),
leading very fast to the unfounded rumor that the Maldivian reefs were dead.
This has led to a bad publicity among divers concerning the Maldives, and
potential visitors may have chosen other destinations for their vacations. 46 %
of the tourists polled at the Male’ airport said that the marine environment
was very important to them (Cesar et al, 2000). With the prediction of global
warming, such disturbances are likely to occur again and actions need to be
taken to mitigate such large-scale events, the source of which is increasingly
thought to be anthropogenic (Glynn, 1991; Brown, 1996; Hoegh-Guldberg, 1999;
Wilkinson, 1999). The natural rate of recovery after a severe disturbance is
slow and it can take five to ten years to recover from a small, localized
disturbance such as a ship grounding and one to several decades for larger
impacts such as coral bleaching (Edwards and Clark, 1998).
To remedy this bad publicity, there is a need
to counter the adverse effects of coral bleaching and other impacts and to
increase the satisfaction of divers. Nowhere in the world is an efficient
method to counter bleaching to be found, as the research in this sector is
still at its beginning. Therefore a research aspect will have to be incorporated
in the project to assess the success and failures as well as to direct the
efforts. Spieler et al. (2001), advocate for a research program to be
incorporated in every single restoration project until a better understanding
of restoration methodology is acquired, as well as a monitoring of the project
for at least a period of 5 years. The results of the research and of the
monitoring undertaken, as well as the directions followed will then be
presented and explained to the interested divers, thereby increasing their
potential satisfaction.
The present enhancement program for the site
looks at a management strategy to integrate human activities such as
recreational diving and pre-emptive reef restoration techniques, in particular
in the case of extensive coral bleaching events. Diverse ecological and
physical aspects of the reef would also be studied to increase the overall
knowledge of the reef ecosystem and increase awareness of the guests.
This project will look at the restoration of
the denuded reefs left after the bleaching events, using corals maricultured in
a nursery. This technique was first developed by Rinkevich in Eilat in the Red
Sea and was called the “gardening of coral reef” (Rinkevich, 1995). In this
technique, different types of coral material is used to create either colonies
or fragments which can then be transplanted on the bare substrate. It will also
investigate the integration in the natural environment of artificial reef
structures, which will be utilized as the nursery. These
artificial structures will consist of several grouped ReefBalls, which are
concrete structures. This implies a monitoring and an understanding not
only of the artificial reef structures but also of the natural conditions of
the target site for the restoration.
Natural reef
Reef processes
Although coral reefs are receiving more and more research focus, they
are still unknown in a number of respects. Studies incorporating the different
aspects of coral reefs are lacking and a holistic view need to be adopted to
understand the reef processes. The physical conditions on the reef determines
to a certain extent the configuration of the benthos, which in turn will
provide certain types of habitat suitable for particular species. The
interactions on the coral reef are very complex and an understanding of the
reef physical, biological and ecological processes in a site such as the Kuda
Huraa House Reef will require observation of diverse aspects in a long term
study. The knowledge of the natural cycles at this site is a target for the
research program and is important for making management decisions.
Physical
environment
Present condition
The site is
characterized by the presence of interruptions in the continuity of the reef
top framework. The presence of this break also corresponds to the area where
the current created by the oceanic swell on the reef crest flows into the
lagoon due to the presence of Kuda Huraa and Vahboahuraa. The back reef slope
also comes to an end at this place and is replaced by a sandy slope forming a
flat area inshore close to the reef top, and sloping outwards until it meets
the lagoon sea floor.
Along this slope
several small patch reefs and isolated bommies are to be found, as well as a
wreck (see map). Two slightly bigger reefs are found to the north and to the
south of the site. The sandy area in between those patch reefs consists of fine
sand and was strewn with dead leaves of seagrass, but it is not known whether
their presence is seasonal. From the aerial picture of the site (Fig. ), the
sand appears to be gathering at the south of the openings of channel connecting
to the reef flat area. The settling of the sand and the distribution of the
leaves are both governed by the hydrodynamic regime of the site.
Hypothetical
processes operating at the site
The first hypothesis is that the presence of
the break in the lee side of the reef and its position between the two islands
are related. The currents created by the oceanic swell setup on the atoll rim
would be flowing in between the islands of Kuda Huraa and Vahboahuraa. The
water flows as if in a funnel and would be increased at the break. The flow
could possibly be responsible for the presence of the break, which is in turn
supported by the shape of the reef itself. The reef crest line is concave in
this area, which induces the idea that the currents would have caused abrasion
of the reef framework, enhancing erosion. All the more that the water coming
from the reef flat would carry sand and debris created on the atoll rim reef
crest.
Where the width of
the reef system is greater, such as near Kanifinolhu or Bodu Huraa, this sand
accumulates in the lagoon. It is possible that the lagoon present to the north
of the reef system was extending southwards in the past, and was filled up
after the reefs caught up with Holocene rising sea levels, but this could only
be proven by cores or the presence of corals in growth position underneath this
sand. A coral microatoll found on the reef flat east of Bodu Huraa was dated
and found to be 710 ± 80 years old, but little can be
inferred from this value and simply proves Gardiner, who assessed the boulder
conglomerates to be 4000 years old wrong (Woodroffe and McLean, 1992). However,
the sand has now filled up this lagoon and the loose sediment created by the
erosion of coral growth on the atoll rim crest passes through the break forming
over time an accumulation on the atoll side of the reef system. The shape of
the gentle sandy slope to the sea floor on the inside of the atoll, with more
sand accumulation to the south of the openings may be explained by a monsoonal
change in the hydrodynamic regime. The hypothesis is two fold, first the
direction of the main flow along the atoll side would be influenced by the
monsoon, and second the sediment supply is also affected by the monsoon. During
the northeast monsoon, the current on the inside of the atoll would flow in a
southwesterly direction and the sediment supply would be high, as the northeast
monsoon wind waves helps the swell created flow to transport the sediment to
the lee side. During the southwest monsoon, the flow would have a northeasterly
direction, and sediment supply would be low, as the monsoon wind waves hinder
sand transport to the lee side.
Another important mechanism that could account
for part of the physical configuration is the action of borers on the blocks of
the upper reef framework, undermining them and making the framework more
fragile. This action would have been enhanced by the swell created flow by
removing the loose sediments in this place.
This in turn could have led the blocks to topple over and fall down the
slope. A similar reef front progradation was observed at the Tin Smelter reef
in Phuket, South Thailand, by the splitting and the toppling of massive porites
colonies and their reestablishment a little further seaward down the fore-reef
slope (Tudhope and Scoffin, 1994). The difference there is that the area is a
sedimentation area where borers are passive endoliths and
vertical splitting of the coral skeleton is facilitated by the abundance of
elongate cryptoendoliths parallel to the growth direction (Scoffin and
Bradshaw, 2000). In our case, this hypothesis would also account for the
concave shape of the reef in that area, (Fig 1), as well as the presence of
coral patches down the slope.
Benthos
Present
condition
At the site, the overall condition of the
benthos was representative of the overall conditions prevailing in the Maldives
since the 1998 coral bleaching event. Few living corals are present on the
upper part of the reef, which exhibits a bare substrate overgrown with turf
algae. The presence of ascidians as well as of coral colonies of the species, Porites
rus, is also common in these conditions.
The coral growth increases with depth, and the
deeper reefs display a good coral cover, with a number of areas dominated by
the genus Goniopora. The patch reefs are dominated by a number of large
colonies of Porites, Diploastrea, Psammocora and Galaxea. A
number of dead colonies form coral heads, which are usually, colonized by an
assemblage of diverse benthic species among which are coral species, usually
encrusting or branching. Areas of rubble are also present and in these cases,
they are often colonized by Acropora species. These colonies are small
in size, and have probably settled subsequent to the 1998-bleaching event.
Whereas seagrass are found in the vicinity of
the island, on both the eastern and the western side, they are absent from the
reef flat directly upstream of the break, to a distance of approximately 500 m.
Nevertheless, a patch of seagrass of around 40 m2 at a depth of 4m
was present downstream of the channels from the reef flat.
The substrate between the patch reefs is sandy,
providing a favourable habitat for numerous sand-dwelling organisms. Blades of
seagrass are found on the sand, which could potentially increase the available
nutrients in the area. Holothurians, which are detritus or filter feeders,
dominated some of the sandy areas. These organisms are important in “cleaning”
the sand and digests bacteria and plankton in the organic matter (Coleman,
2000).
Hypothetical
processes operating at the site
The benthic composition of a reef is influenced
by the physical conditions, like currents influencing recruitment, or
sedimentation hindering coral growth. Therefore, the benthic species
composition, the condition of the colonies and their shapes will give clues
about the physical factors and their influences.
At the Kuda Hura House Reef, the oceanic swell
created flow could play an important role on the ecology of the reef by
promoting the exchange of species from the outer reef crest to the inside of
the atoll. It could also be bringing food to the site, in the form of plankton from the reef flat
and crest. Import of species from upstream could also
be responsible for the presence of the seagrass patch. The presence of
seagrass on the reef flat near Kuda Huraa is possibly due to increased nutrient
input into the area as a result of human habitation. The aerial pictures from
1969, prior to resort development does not show any seagrass near Kuda Hura.
However, since the establishment of the resort, seagrass has been seen as a
problem for the aesthetics of the island. A study carried out in Laamu Atoll
found that seagrass was related to an increase in the input of phosphorous into
the lagoon (Miller and Sluka, 1999). Phosphorous is one of the common nutrients
that are found in the human effluent and thus it is not surprising that the
presence of seagrass in the Kuda Hura lagoon has been observed since the
habitation of the island. Whether the increase in nutrient levels in the water
has had an influence on the benthic communities is not known, and would be
difficult to determine as the effects would have been masked by the 1998 coral
bleaching event.
Coral bleaching occurs when corals lose or
expel a major portion of their zooxanthellae, when the concentration of the
photosysnthetic pigments in the zooxanthellae decreases severely or due to a
combination of these processes. It is a stress reaction that can be induced by
certain stressor including elevated or decreased water temperatures, high
fluxes of visible and ultraviolet radiation, prolonged aerial exposure,
freshwater dilution and high sedimentation (Glynn, 1991). Responses to
bleaching include reduced coral growth and calcification, diminished capacity
to reproduce and tissue necrosis. Intense and prolonged bleaching however can
cause high coral mortality (Glynn, 1993).
When bleached corals die, the available space
is often colonized by non-reef-building organisms. The dead framework act as
shelter and grazing surfaces for many potentially destructive organisms such as
boring sponges, mussels, sea urchins and fish, leading to higher bioerosion than
net carbonate production (Glynn, 1991).
Some coral species are more susceptible to
coral bleaching than others. Corals having massive morphologies have been found
to be less affected while those with branching morphologies are more
susceptible. Marshall and Baird (2000) found that there were significant
differences in bleaching response between depths and taxa. Cyphastrea,
Turbinaria and Galaxea were relatively unaffected by bleaching,
while most acroporids and pocilloporids were highly susceptible. The hydrocorals
(Millepora spp.) were the most susceptible taxa, with 85% mortality.
Before the 1998 bleaching event, many reef flats in Maldives were dominated by
branching acroporids. As these species have very low susceptibility to
prolonged elevated temperatures, high mortality occurred during this event.
Many hardy corals such as Porites lutea, P. cylindrica and P. rus
and Goniopora spp. seem to have survived the bleaching event.
Recruits of Acropora and Pocillopora in
the 30cm size class were observed at the Kuda Hura House Reef especially in the
area of dead coral rubble on the southernmost patch reef. Considering
their size, these colonies have probably recruited subsequent to the bleaching
event. Some species such as Stylophora pistillata and Seriatopora
hystrix, which were common pre-bleaching but are thought to have
disappeared from the northern atolls, were absent at the site.
The structure of the benthic community is an
important factor shaping the reef ecosystem. It provides habitats for a large
number of fish and mobile invertebrates, which depend on the reef framework for
shelter. The way the benthic communities influence the distribution of habitats
for the mobile fauna will be a focus of this study (e.g. affinity of certain
fish species for certain coral forms or species). The assemblages of fish and
invertebrates found on the reef can reciprocally affect the benthos. For
example, some species of parrotfishes which are herbivorous fish grazing on
algae excavate the substrate, feeding predominantly in the shallow part of the
reef, with preferential grazing on convex surfaces such as dead coral stumps.
Some of these species have been observed to defecate mainly in deeper areas
such as gullies and over the reef base As they primarily feed on convex surfaces
and defecate in depressions, they have the potential to reduce the overall
topographic rugosity of the reef top (Bellwood, 1995).
Coral polyps, tunicates, sponges, algae and
other sessile animals are a source of food for a number of species. Two species
of obligate corallivores, Gobiodon citrinus and Oxymonacanthus
longirostris, which were common on Maldivian reefs pre-bleaching have
virtually disappeared with the death of the branching acroporids which provided
shelter and food for these species.
Fish
and mobile invertebrates
Settings
The diversity of fish observed at the Kuda Hura
House Reef was very high with 141 species belonging to 90 genus being recorded
from the area. The fish community appears to be healthy with an abundance of
predators such as groupers, snappers, moray eels and scorpion fish. Cryptic
species like Calloplesiops altivelis, were seen swimming freely near
their crevices. Small carnivores such as apogonids were very frequent,
gathering in schools in sheltered space.
Another characteristic of the site is the
presence of schools of balistids, such as Odonus niger in the water
column which are usually not found in back reef
conditions, and prefer the currents of the channels. A large number of Pseudobalistes
flavimarginatus, showing a schooling behaviour was also present.
Omnivores are also well represented at the
site, with a number of pomacentrids, chaetodontids, blennids, gobiids and
pomacanthids. Among those families, species like Apolemichtys xanthurus,
Chaetodon decussatus or Gunnelichtys curiosus, which are quite
rarely encountered were sighted.
Herbivorous fish were not lacking either, with
all the major families including acanthurids, scarids, kyphosids and siganids
present. It appears that in each of those families, large size individuals were
frequent. The variety of resources enables the presence of rare species, such
as the ornate ghost pipefish Cheilodipterus artus.
This diverse fish fauna also sustains a number
of fish associated species such as shrimps. Four species of shrimps were
recorded from the site Two of these species Urocardiella antonbruunii
and Rhynchocinetes durbanensis had set up some cleaning stations, while Stenopus
hispidus was also seen and is well known to clean big predators like moray
eels. Other invertebrate fauna include the octopus Octopus cyanea, a
large number of royal sea cucumber, Thelenota anax, on the sandy sea
floor, and a few spiny lobsters Panulirus versicolor.
Hypothetical processes operating at the site
The site seems is
obviously characterized by a diversity of resources. To simplify, resources are
usually divided into two broad categories, food and living space (Sale, 1980).
The presence of patch reefs down the slope separated by sandy areas provides at
least two distinct habitats, which sustain two different communities. The
diversity at the spot is therefore enhanced by the physical settings.
In the scientific literature, evidence that
food directly limits number of fish is limited (Sale, 1980), and it is
therefore difficult to find grounds for the null hypothesis of this general
rule. In our case though, the species richness, the size of the individuals as
well as the abundance of secondary transformer tends to show that the food
resource is excellent at the site. As mentioned earlier, the role of the
current flowing over the reef flat is probably important, but medium to
long-term observations are required to establish the interactions within and
between these communities. The study concerning the fish and the mobile
invertebrates will try and determine the relative roles of competition,
predation and abiotic factors in structuring the fish and mobile invertebrate
communities.
There are a number of possible theories
explaining the structures of fish communities. They can be divided into two
classes. The “equilibrium” theories state that there is an equilibrium between
the amount of resources like food and shelter and the fish population, and that
this limits the number of fish as a whole. The other class, regrouping the
“non-equilibrium” theories, does not make the assumption that the amount of
resources is a limiting factor for the fish population. Instead they propose
that the limiting factor for the fish population would occur during recruitment
(Victor, 1983, 1986; Doherty and Fowler, 1994), despite the prodigious
fecundity of fishes (Sale, 1977, 1978).
There are two strands among the supporters of the “equilibrium
theories”. The first school of thought favours the “single-species
equilibrium”. In this model, the community evolved to create species-specific
niches, and therefore, the competition for the limited amount of resources
would mostly occur between conspecifics. The second line of thought called
“multi-species equilibrium” suggests that similar species would compete for the
same resources. The amount of available resources would then determine the
total amount of fish, but not the number of individuals in each species.
One last pluralistic theory states that any of the above mentioned
models could take precedence in a reef at a given time as the relative
population abundances of fish are determined by temporal and spatial variations
in recruitment strength, immigration and emigration, predation and competition
for shelter sites (Caley, 1993). Therefore it is to be classified among the
“non equilibrium theories”.
In order to decide which model is closest to
reality, the evolution of the fish community will be followed and compared with
the predictions of the different models. A highly fluctuating community
structure, as noticed by different researchers in Australia (Sale and Dybdahl,
1975, 1978; Sale, 1977; Talbot et al., 1978; Sale and Douglas, 1984; Sale et
al., 1994), is in favour of either a non-equilibrium theory, or the
“multi-species equilibrium”, whereas constant community structure, as noticed
in the Caribbean (Ogden and Ebersole, 1981), would favour the “single species
equilibrium”. Wantiez and Thollot noticed interspecific competition on some
50-year-old artificial structures in New Caledonia, together with post settlement
migration of highly sedentary damsel fishes, thus favouring the single species
equilibrium.
A monitoring of the
reef using a fish count is required over a period of time in order to determine
which of these models is applicable to the present situation.
Artificial reefs
Concept of restoration
Coral reefs worldwide are in a state of decline
from natural (i.e. storms, predator outbreaks, coral bleaching etc.) as well as
anthropogenic causes such as coastal development, pollution and exploitation (Wilkinson,
1999). Unlike in terrestrial environments, the impacts in marine systems are
not localized due to high connectivity through ocean currents (Allison et
al., 1998). Damage due to natural
causes is often widespread and catastrophic and human intervention or
restoration is not feasible. However, anthropogenic damage can be prevented by
mitigating the impacts and rehabilitating or restoring impacted environments.
Artificial substrates have recently become a highly used tool for accomplishing
such goals (Spieler et al., 2001). Reef restoration is largely limited
by incomplete knowledge of the ecosystem processes (Clark, 2001; Spieler et
al., 2001). It is therefore important to build up this knowledge as the project
unfolds.
When attempting to restore a reef impacted by
coral bleaching and recreational diving, (the two stresses that have been
identified as being the major cause for concern in our situation), it is very
important to identify the functions that artificial reef structures can have.
In some studies, where the reef framework is lost, as in the case of a ship
grounding or coral mining, concrete can be used to replace the reef framework
in the short term (Spieler et al., 2001). In the case of coral bleaching
however, the reef framework is left intact, if
we exclude areas of very large stands of Acropora species of the tree type
(erect, usually branching projections with a restricted zone of substratum
attachment) like A. cervicornis or A. Formosa, which are reduced
to unconsolidated rubble. This rubble is usually
unstable and not a favourable substratum for the survival of the recruits,
which settle on it. In the long term, coralline algae and internal
sedimentation usually consolidate and cement this rubble. Divers mostly impact
the more fragile foliose or branching forms, and do not have a significant
impact on the reef framework.
Thus, it is understood that the problems faced
is the recolonization of the bare substrate by coral species rather than a lack
of substrate itself. Natural recruitment is judged to be insufficient for rapid
recovery at a human scale (Edwards and Clark, 1998), and therefore this natural
process can be accelerated by human intervention, with benefits in certain
cases such as the degradation of a popular dive site. This can be achieved
through transplantation of corals to enhance recovery.
Edwards and Clark
(1998) reviewed the projects, which have been carried out on coral reef restoration and the reasons stated for
coral transplantation were to 1) accelerate reef recovery after ship
groundings, 2) replace corals killed by sewage, thermal effluents or other
pollutants, 3) save coral communities or locally rare species threatened by
pollution, land reclamation or pier construction, 4) accelerate recovery of
reefs after damage by Crown-of-thorns starfish or red tides, 5) aid recovery of
reefs following dynamite fishing or coral quarrying, 6) mitigate damage caused
by tourists engaged in water-based recreational activities, and 7) enhance the
attractiveness of underwater habitat in tourism areas. The reasons 6 and 7
apply to the present project, which also has an additional pre-emptive aspect
in anticipation of a coral bleaching event. So far, cases of pre-emptive
restoration include mostly rescue and relocation of corals threatened by human
activities (Clark, 2001).
The aim of the
program is to enhance the recovery of the Kuda Hura House Reef from bleaching
events and diver damage. The objectives are to try
in a first phase, to grow coral colonies in nursery areas, which would be less
impacted by a coral bleaching event, so that afterwards, the recruitment on the
bare substrate can be supported by the transplant of fragments pruned off the
colonies grown in the nursery areas.
These types of nurseries have already been
tested in the Red Sea for Stylophora pistillata, a technique called
“gardening coral reefs” (Rinkevich 1995; Rinkevich 2000; Epstein et al, 2001).
The different coral material experimented on were
fragments, small colonies, coral larvae and coral nubbins, which are fragments
of coral colonies consisting only of a few polyps. The two latter ones have
been concluded to be expensive techniques not suitable in our case where
logistics and manpower would not be sufficient and thus will not be utilised.
Small coral colonies will not be taken for transplantation, as they may be
recruiting in an area suitable for their growth. Broken fragments of corals are
available on the site, either due to natural breakage or from impacts of diving
activities, and these will be the preferred choice for transplantation onto the
ReefBalls.
Some nurseries will be deployed in areas deep
enough to be protected from a bleaching event. Even though deep areas are less
affected by higher than normal temperatures, some of these artificial reefs will
be deployed at different depths, including relatively shallow areas. This is in
part to test the resistance of corals to changes in depth.
In case not enough fragments are available at
the site on that day, fragments will be pruned off selected colonies. These
colonies will be healthy colonies showing a good phenotype, like fast growth
(larger colonies), or adapted to higher temperature (from shallow zones). Some
studies have been carried out on the optimal pruning levels and pruning more
than 10 % of the branches of a donor coral colony of Stylophora pistillata
has been shown to decrease survival and reproductive activity of the donor
colonies (Epstein et al, 2001). In contrast to Stylophora pistillata,
acroporid species are known to reproduce asexually by this method, and a limited number of fragments will have to be
collected to avoid damaging the donor colonies as well as to avoid reducing the
genetic heterogeneity of the genetic pool (Epstein et al, 2001). The details of
the deployment and the monitoring of the experiment will be explained in a
later paragraph. An in-situ “nursery period” superior to 5 years is predicted
for S. pistillata small fragments (Rinkevich, 2000), and again, this
period may be shorter might be reduced in the case of acroporids, as asexual
reproduction is a natural phenomenon for these species.
A number of projects and studies using coral
transplantation have shown some encouraging successes, (e.g. Guzmán, 1991;
Bowden-Kerby; 1997; Muñoz-Chagin, 1997; Oren & Benayahu, 1997; Lindahl,
1998, Smith and Hughes, 1998). Based on these studies, as well as their own
experience in the Maldives, Edwards & Clark evolved a guideline for coral
transplantation (Edwards and Clark, 1998), which is good to consider when
planning this type of activity. The major obstacles encountered by researchers
stated in their study are: bad water quality, stability of substratum, and
presence of a ready supply of donor colonies and fragments. Water quality would
not be changed in our case, as the ReefBalls are made of a stabilised concrete,
which do not affect it. The coral reef framework left bare after the bleaching
provides a stable substratum and thus the stability of substratum will not be
an issue. As for the last point, a few
colonies will be pruned in the first part of the program, in case not enough
broken coral fragments corals are found, but it should be noted that the aim of the study is
itself to create a ready supply of donor colonies for a larger operation at a
more critical period when the need arises.
The restoration of the reef by this method is
only one a part of the project, as it is
well understood that the knowledge of the natural environment, its processes
and its community structure before the impact is necessary in order to assess
the success of the restoration. Reef recovery can be defined as recovery
of the reef to its pre-disturbance state. With a localized impact, recovery can
be assessed by comparing with adjacent undamaged areas. However when the impact
is large-scale such as with a bleaching event, it is difficult to determine
when a reef has recovered, especially if data on pre-bleaching status is
lacking. Can we talk about reef recovery if the coral
communities exhibit high live coral cover but low species diversity? For example in Florida, reef recovery in terms
of live coral cover was achieved within 5 years although the community was
dominated by just one species (Shinn, 1976). It is therefore crucial to know
what the ecological state of the habitat and of the communities was prior
to the disturbance, which is often lacking in many reef restoration projects
(Clark, 2001). A good knowledge of the benthic communities is a key factor in
this program, and mapping the present reef as thoroughly as logistically possible will be of great help in order to
preserve the structure of the benthic community in the recovery effort after
any future disturbances. This point is further discussed in a later paragraph.
When deploying artificial reef structures,
their impacts on the already existing community of the nearby reef must be
given as much importance as the engineering aspects including composition,
texture, size and stability of the artificial structure. This consideration is
important for both the benthic species and the mobile marine life. The debate
between “attraction”, when fishes from the nearby reef migrate to the
artificial structures, and “production” when the artificial structures help
sustain a larger fish population, as in the case of FADs (Fish aggregating
devices), is trying to assess the role of artificial reefs.
Engineering
considerations
The Reef Balls that will be utilised in the
present project are the ones of the model Bay Ball
with a diameter of 4feet. These Reef Balls will
be constructed in concrete on the island with a mould acquired by the Four
Seasons. The concrete used is a stable structure, which has a rough surface
texture, and which will be beneficial to natural recruitment, as it suits
scleractinian corals well (Spieler et al., 2001). The concrete is itself stable and has not been reported to alter
water quality in its vicinity.
The Reef Balls have a hemispherical shape with
a hollow inside and a number of circular openings between the outside and the
inside chamber. The number of openings can be chosen, and an increased number
of holes increase the ratio of the surface area over the mass. This parameter
is of important consideration, as the increased complexity of the structure
will increase shelter space. A total of x will provide a ratio between the mass
and the surface area of y, and this parameter will be fixed, so that each reef
can be used as a replicate for the measures done, and will not introduce a bias
in the statistics.
The shape of the Reef Balls allow for a low
centre of gravity, and therefore these structures are fairly stable, and have
been used in breaking waves without toppling over. The hydrodynamic conditions
at the sites chosen are not this challenging in any way, but deployment of the
Reef Balls will be done in the flatter parts of the sandy areas to avoid
slipping.
The reef balls will be deployed by groups of
10, which will be considered replicate artificial reefs in the scientific
protocols. These 10 reef balls will be deployed in
three rows, one of four and two of three on either side of the first, according
to the following design.
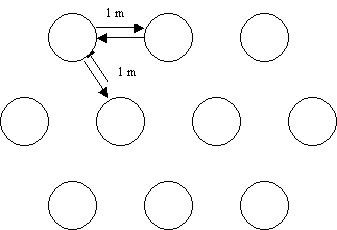
The surface area of
these reefs is 30m2. A distance of 1 m is left between the reef
balls to allow for coral growth on each side sides.
The last
engineering consideration is probably what makes most
of the success of the reef balls: the ease of deployment. The reef balls are
very easy to deploy as an inflatable ball enables the control of its buoyancy.
They can be towed behind a boat to the site, and then brought to the desired
spot while diving using SCUBA. This method, and the sites chosen for deployment
far from any coral growth guarantee that the deployment will be done without
any risk of damage to the pre-existing
environment.
Benthic
The colonization of
the artificial structures by benthic species is of great concern, as this will
enable their blending into the natural reef landscape. There is a pattern of
succession by different groups of organisms as they colonize a substrate, which
depend on their particular strategies and the external conditions. The focus of
the project is on corals as has been emphasized earlier, and the research
effort will focus on ways to enhance the colonization of this group over the
others.
Edwards and Clark (1998) have critically
examined current transplantation approaches and concluded that in most cases,
where the degraded area receives sufficient recruits naturally, coral
transplantation should be the last resort of a restoration effort. Further,
they suggested that slow-recruiting, slow growing massive corals be used when
coral transplantation is justified. Spieler et al. (2001) retorted that
these conclusions highlight the need for an artificial structure, at least in
the short term, to replace the structural function of these corals. Indeed, in
their restoration program, Edwards and Clark (1998) were looking at the
restoration of a reef heavily mined and where the reef framework had been
completely removed, leaving only loose sediment on the surface. Thus, their
conclusions are far from being applicable to all artificial reef structures
projects. Typically in the present project that focuses on a bleached reef it
is preferable to culture in the nursery corals species which are the most
affected by bleaching, such as the ones belonging to the genus Acropora or
Pocillopora. In many instances, fast growing, branching coral species
with high metabolic rates were among the first to bleach and die (Glynn, 1992).
In the same direction, species of the genus Seriatopora, which have been
seriously reduced during the 1998 bleaching events and are now commonly seen
only in Addu (Hussein Zahir, pers. comm.), present a lot of interest. These
fast growing species have already been the subject of most of the research in
using coral fragments to reproduce species asexually. Another reason to focus
the efforts on these species is that the branching and foliose types of corals
are more sensitive to recreational diving than the massive ones.
Preferentially, broken coral fragments found lying will be used for coral
transplant onto the reef balls.
The broken fragments will be attached to plugs
set in the reef ball structure using epoxy resin. Attached fragments show a
better survival rate than loose fragments, which are often lost or abraded (Smith and Hughes, 1999). The artificial substrate
will also be the object of natural recruitment of other species such as algae
and ascidians, which are fast settlers. An area around half the coral plugs
will be brushed regularly to investigate the survival rate of corals subject to
lower competition levels. Natural recruitment of corals onto the reef balls
will also be followed and thus, the benefits of human intervention can be
quantified.
Colonization by
fish
The colonization of artificial reef structures
by fish species has been the subject of a number of studies (Klima and Wickham,
1971; Shulman et al., 1983; Tupper and Hunte, 1998; Sherman et al. 1999;
Wantiez and Thollot, 2000). These studies show that the size of the artificial
structures, as well as its shape has great importance in determining both the
species composition and the stability of the community structure.
Klima and Wickham (1971) reported that the hull
of a sunken vessel can cause the aggregation of several species of fish, which
are not typical in the area. Therefore artificial reefs of large size would
perhaps increase the species diversity. On the other hand, small artificial
reefs, isolated by sand or seagrass from natural reefs, attract a fish fauna
similar to that found on small isolated patch reefs (Talbot et al., 1978). The
artificial reef structures, which will be deployed on the Kuda Huraa House
Reef, which will consist of 10 reef balls can be considered as being of middle
size compared to the ones described (ships, cars, Reef Balls, cement blocks
etc.), but the community is expected to be similar to the one of a small
isolated patch reefs.
Several researchers have suggested that the
number of species on a reef will be a function of its size, and that the more
species from a given species pool that the reef can attract, the more similar
will be the fish assemblages on the reef (Ogden and Ebersole, 1981; Sale and
Douglas, 1984; Tupper and Hunte, 1983). Given the size of the artificial reefs
studied reviewed by Tupper and Hunte (0.25m2 to 1m2, 1m2,
8.25m2, 100m2), it appears that the size of 30m2
structures planned for the artificial reefs in our study, should show some
similarity in time, promising some interesting results.
Wantiez and Thollot (2000) found that the
repartition of fish on a given artificial structure depended a lot on the
geometry of the structure, with complicated areas providing shelters enhancing
fish recruitment: if a fish cannot find a shelter when predators attack, it
will invariably be eaten. The Reef Balls have a design providing shelters for
species, and in the long-term, this aspect can be compared with the different
surrounding natural habitats. It is also expected that reef balls with coral
colonies growing on them will provide more shelters than bare ones.
Dive site
Impacts
of recreational diving
There are limits to how many divers a reef can
take without degradation. Dixon et al. (1993) suggest a critical level of about
4500 dives per year before diver impact becomes apparent. In Eilat, despite the
tight legislation and management measures that have been employed for years,
the reef recovery does not sufficiently compensate for the intense destruction
by recreational activities (Epstein et al., 2001). This conflict between
recreational diving and the natural environment the sport relies on, is the
concern of a large body of literature. Davis and Tisdell (1995) argue that
there are critical and biological thresholds above which amenity values are
reduced severely, while biological degradation may also become significant. The interrelationships between amenity and
biological values are worthy of further research to identify biological and
social carrying capacities to formulate suitable management responses to
reduced recreational values of the site.
In this context, education has a significant
role to play by increasing environmental awareness and reducing the damaging
impacts caused by users of the site. The value of the research that will be
carried out will therefore be two fold: it increases the recreational aspect of
the diving experience, and it helps in maintaining the site in good biological
conditions.
Furthermore, using the broken fragments can
mitigate localized noticeable impacts caused by a clumsy diver breaking a
coral. In case the colony has been severely damaged, a fragment of the same
species can be transplanted in its place.
It is also expected that the creation of dive
trails will reduce impacts to the coral reef, enabling the divers to take the
trail suited to their level of experience, i.e., taking
the least experienced ones in less sensitive
areas of the site, while showing them aspects of the reef that often go
unnoticed.
Dive trails
Dive trails should
be designed in cooperation with the dive school.
Certain features of the reefs can be incorporated in the dive trail so
that they can be explained beforehand to the divers. Betti, you probably have
your own routines and therefore it is important that you participate a lot in
this. Apart for the points where the fish species have been identified and the
coral colonies described in the mapping of the reef, there is a few interesting
features that I have noticed:
-
a
cleaning station of Anton Bruuni cleaner shrimps (Urocardiella antonbruunii)
in A (in the fracture of the big bommy) visited by some predators (groupers
seen). These stations are similar to the ones set by the cleaner wrasse (Labroides
dimidiata) but are not as common. In A as well, a possum wrasse (Wetmerolla
nigropinnata) not often seen because quite secretive and rare among the
branches of the psammacora bommy.
-
The
garden eels (Heteroconger hassi) on the sandy areas, which are quite
abundant.
-
A
spiny lobster Panulirus versicolor near coral 22 (underneath, see photo)
-
In
that same area a not so common angel fish Apolemichtys xanthurus.
-
In G,
the ornate ghost pipe fish under the Acropora growing on the deeper side of the
porites bommy, you probably know about this one (I was told by the captain). A
striped pipe fish in the same area, as well as a couple of Calloplesiops
altivelis around the bommy, which are usually secretive and not often seen but
are almost in the open there.
-
A
number of shrimps some unidentified, and banded boxer shrimp (Stenopus
hispidus) near isolated coral head 31.
-
Some
durban hinge-beak shrimp (Rhynchocinetes durbanensis) near the wreck, which
jump on your hands as you approach them, even though they are not specifically
none to set up cleaning station.
-
A pipe
fish not far from I, which is very easily taken for a stick and whose name I
cannot recall (my fish book sadly stayed in Maldives) see picture.
-
There
was a quite rare goby in J (I think it is called the laser blue goby, my
picture of it is bad and I am not sending it), but it was not seen in the
following visits.
Sightings
As part of the
project, it is planned to record sightings of rare and flagship species, which
visit the reef. Initially the different individuals of the flagship species visiting the
reef should be identified. During our visit we have seen two turtles, a fairly
large one, as well as a smaller one. Apart from turtles, there may be some
Napoleon wrasse, which visit and usually attract the attention of visitors. It
should be possible to identify markings on frequently seen individuals and give
them names. The data to be collected, which will be further described in the scientific
protocols, will enable the following of their habits. This has to be done
jointly with the dive school instructors as they would be doing most of the
observations. This data will be entered into the database for processing.
Betti, with the help of the dive school people, do you think you can make a
list of the possible individuals to follow and identify markings on them?
Activities
of the project (will include the scientific protocol)
The surveying and monitoring of the site will
use a method based on an inductive approach, and will consist in mapping the
reefs according to a protocol using GIS (Geographical Information System) for
treating the data. This approach is more adapted than the usual line intercept
transect (LIT) treated with biometry for a number of reasons. One shortcoming
of the LIT method is in its inability of describing the physical settings of a
reef, especially in our case, where there are a number of isolated bommies,
which represent different habitats. A
precise knowledge of what species is living in which place is important for the
achievement of restoration. The LIT method tries to describe the reef from a
random sampling, and therefore does not seem adapted in our configuration,
where exact positions of species would be required. LIT data is hard to
interpret, even for a scientist, let alone the interested tourists, for whom a
graphical representation is much more attractive.
The mapping of the reef will encompass three
aspects, the reef processes like hydrodynamic regime and sedimentation, the
benthic cover, with location of as many coral colonies and species dominated
areas as possible, and the fish and invertebrate life. The ecological links
between these components will be of foremost importance to understand the reef
as a whole.
References
Allison, G.W., Lubchenco, J. and
M.H. Carr. (1998). Marine Reserves are necessary but not sufficient for marine
conservation. Ecological Applications 8(1) Suppl: S79-S92.
Bellwood, D.R. (1995). Carbonate
transport and within-reef patterns of bioerosion and sediment release by
parrotfishes (family Scaridae) on the Great Barrier Reef. Marine Ecology
Progress Series 117: 127-136.
Bouchon, C.. Jaubert, J. and Bouchon-Navaro, Y. (1981). Evolution of a semi-artificial reef built by transplanting coral heads. Tethys 10(2): 173-176.
Bowden-Kerby, A. (1997). Coral transplantation in sheltered habitats using unattached fragments and cultured colonies. Proceedings of the 8th International Coral Reef Symposium 2, 2063-2068.
Brown, B.E. (1997). Coral bleaching: causes and consequences. Coral Reefs 16 Suppl: S129-S138.
Caley, M.J. (1993). Predation,
recruitment and the dynamics of communities of coral-reef fishes. Marine
Biology 117: 33-43.
Cesar et al. ….
Clark, S. (2001). Volume II, Chapter
8: Coral Reefs. In: ? (ed). The Handbook of Restoration Ecology. ??
Coleman, N.
(2000). Marine life of the Maldives. Atoll Editions, Australia. 326pp.
Davis, D. and
Tisdell, C. (1995). Recreational scuba-diving and carrying capacity in marine protected areas. Ocean
& Coastal Management 26(1): 19-40.
Doherty, P.J. and Fowler, A. (1994).
An empirical test of recruitment limitation in a coral reef fish. Science
263: 935-939.
Edwards, A.J. and Clark S. (1998). Coral transplantation: a useful management tool or misguided meddling? Marine Pollution Bulletin 37: 8 – 12.
Epstein, N., Bak, R.P.M. and Rinkevich, B. (2001). Strategies for Gardenning Denuded Coral Reef Areas: The Applicability of Using Different Types of Coral Material for Reef Restoration. Restoration Ecology 9 (4): 432-442.
Glynn, P.W. (1991). Coral Reef bleaching in the 1980s and possible connections with global warming. TREE 6(6): 175-179.
Glynn, P.W. (1993). Coral Reef bleaching: ecological perspectives. Coral Reefs 12: 1- 17
Gutierrez, L. (1998). Habitat selection by recruits establishes local patterns of adult distribution in two species of damselfishes: Stegastes dorsopunicans and S. planifrons. Oecologia 115: 268-277.
Guzmán, H.M. (1991). Restoration of coral reefs in Pacific
Costa Rica. Conservation Biology 5, 189-195.
Hoegh-Guldberg, O. (1999). Climate change, coral bleaching and the future of the world’s coral reefs. Marine and Freshwater Research 50: 839-866.
Hughes, T.P. (1994). Catastrophes, Phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265: 1547-1551.
Klima, E.F. and Wickham, D.A. (1971). Attraction of coastal pelagic fishes with artificial structures. Transactions of the American Fish Society 100: 86-89.
Lindahl, U. (1998). Low-tech rehabilitation of degraded coral reefs through transplantation of staghorn corals. Ambio 27 (8), 645-650.
Loehle, C. (1990). A guide to increased creativity in research – Inspiration or perspiration? BioScience 40: 123-129.
Marshall, P.A. and A.H. Baird.
(2000). Bleaching of corals on the Great Barrier
Reef: Differential susceptibilities among taxa. Coral Reefs 19(2):
155-163
Miller, M.W. and R.D. Sluka. (1999).
Patterns of seagrass and sediment nutrient distribution suggest anthropogenic
enrichment in Laamu Atoll, Republic of Maldives. Marine Pollution Bulletin.
38(12): 1152-1156.
Muñoz-Chagin,
R.F. (1997). Coral transplantation program in the Paraiso coral reef, Cozumel
Island, Mexico. Proceedings of the 8th
International Coral Reef Symposium 2,
2075-207
Ogden, J.C. and J.P. Ebersole.
(1981). Scale and community structure of coral reef fishes: a long-term study
of a large artificial reef. Marine Ecology Progress Series 4:
97-103.
Oren, U. & Benayahu, Y. (1997). Transplantation of
juvenile corals: a new approach for enhancing colonization of artificial reefs.
Marine Biology 127, 499-505.
Rinkevich, B. (1995).
Restoration strategies for coral reefs damaged by recreational activities: the
use of sexual and asexual recruits. Restoration
Ecolology 3 (4): 241-251.
Rinkevich, B. (2000). Steps towards the evaluation of coral reef restoration by using small branch fragments. Marine Biology 136: 807-812.
Sale, P.F. (1977). Maintenance of high diversity in coral reef fish communities. American Naturalist 111: 337-359.
Sale, P.F. (1978). Coexistence of coral reef fishes – a lottery for living space. Environmental Biology of Fishes 3: 85-102.
Sale, P.F. (1980). The ecology of fishes on coral reefs. Oceanography and Marine Biology Annual Review 18: 367-421.
Sale, P.F. and Douglas, W.A. (1984). Temporal variability in the community structure of fish on coral patch reefs and the relation of community structure to Reef Structure. Ecology 65 (2): 409 – 422.
Sale, P.F. and R. Dybdahl.
Scoffin, T.P. and Bradshaw, C. (2000). The taphonomic significance of endoliths in dead – versus live – coral skeletons. Palaios 15: 248-254.
Sherman, R. L., Gilliam, D. S. and Spieler, R. E. (1999). A preliminary examination of depth associated spatial variation in fish assemblages on small artificial reefs. Journal of Applied Ichthyology 15 (3): 116
Shinn, E.A. (1976). Coral reef recovery in Florida and the Persian Gulf. Environmental Geology 1, 241-254.
Schulman, M.J., Ogden, J.C., Ebersole, J.P., MacFarland, W.N, Miller, S.L. and Wolf, N.G. (1983). Priority effects in the recruitment of juvenile coral reef fish. Ecology 64 (6): 1508-1513.
Smith, L.D. and Hughes, T.P. (1999). An experimental assessment of survival, re-attachment and fecundity of coral fragments. Journal of Experimental Marine Biology and Ecology 235: 147 – 164.
Spieler, E.S., Gilliam, D.S. and Sherman, R.L. (2001). Artificial substrate and coral reef restoration: What do we need to know to know what we need. Bulletin of Marine Science 69 (2): 1013-1030.
Talbot, F.H., Russell, B.C. and Anderson, G.R.V. (1978). Coral reef fish communities: unstable, high diversity systems? Ecological Monographs 48: 425-440.
Tudhope, A.W., Scoffin, T.P, 1994, Growth and structure of fringing reefs in a muddy environment, South Thailand. Journal of Sedimentary Research A64: 752-764.
Tupper, S. and Hunte, W. (1998). Predictability of fish assemblages on artificial and natural reefs in Barbados. Bulletin of Marine Science 62 (3): 919-935.
Victor, B.C. (1983). Recruitment and population dynamics of a coral reef fish. Science 219: 419-420.
Victor, B.C. (1986). Larval settlement and juvenile mortality in a recruitment-limited coral reef fish population. Ecological Monographs 56: 145-160.
Wantiez, L. and Thollot, P. (2000). Settlement, post-settlement mortality and growth of the damselfish Chromis fumea (Pisces: Pomacentridae) on two artificial reefs in New Caledonia (south-west Pacific Ocean). Journal of the Marine Biological Association of the United Kingdom 80: 1111-1118.
Wilhelmsson, D., Ohman, M., Stahl, H. and Shlesinger, Y. (1998). Artificial Reefs and Dive Tourism in Eilat, Israel. Ambio 27 (8): 764 – 766.
Wilkinson, C.R. (1999). Global and local threats to coral reef functioning and existence: review and predictions. Marine and Freshwater Research 50: 867-878.
Woodroffe, C. and McLean, R.F. (1992). Assessment of recent sea-level change on Pacific and Indian Ocean Atolls: deciphering the record from microatolls. Unpublished report.







 Appendix
1: MAP OF REEF BALL PLUGS
Appendix
1: MAP OF REEF BALL PLUGS
REEF BALL 1
 REEF
BALL 2
REEF
BALL 2 







REEF BALL 3
REEF BALL 4
KEY TO REEF BALL CORAL SPECIES

ACROPORIDAE

ACROPORIDAE
/ POCILLOPORIDAE

PORITIDAE

OCULINIDAE / DENDROPHYLLIDAE

EMPTY

 DEAD
DEAD
DYING 12.8.02
Appendix 2: Excel Data
|
DATE |
WEATHER |
TEMP |
RB No. |
DEPTH |
POSITION |
RB OBS |
PLUGS OBS |
TOTAL No. |
EMPTY |
HEALTHY |
DYING |
DEAD |
MARINE LIFE |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tuesday |
Sea Calm |
|
1 |
7.1 |
Close to Buoy |
Ball Disturbed |
|
11 |
1,9 |
|
|
2,3 |
Labridae, Pomacentridae |
|
|
||||||||||||||
|
11/20/01 |
No Wind |
|
|
|
|
Probably by divers |
|
|
|
|
|
|
Cirrithidae |
|
|
||||||||||||||
|
Morning |
|
|
2 |
7.6 |
Close to buoy |
|
|
8 |
|
|
|
3,4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
and RB 1 |
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
3 |
9 |
Left of RB1 & 2 |
|
|
12 |
|
|
|
5,12 |
|
|
|
||||||||||||||
|
|
|
|
|
|
near coral blocks |
|
|
|
|
|
|
possibly 1,3,4 |
|
|
|
||||||||||||||
|
|
|
|
4 |
11 |
sandy slope |
|
|
12 |
|
|
|
2 |
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Tuesday |
|
|
1 |
8.7 |
RB 1&2 moved |
RB 1&2 need to be |
|
|
1,4,6,7,9 |
|
|
|
|
|
|||||||||||||||
|
11/27/01 |
|
|
|
|
close to RB 3. |
removed to a flat |
|
|
|
|
|
|
|
|
|||||||||||||||
|
Morning |
|
|
|
|
On slope and seem |
surface |
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
unstable, leaning close |
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
to other coral blocks |
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
2 |
9.6 |
|
|
|
|
|
|
|
3 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Thursday |
|
|
1 |
|
|
photographs |
New fragments |
|
1,4,6,7,9 |
|
|
|
|
|
|||||||||||||||
|
12/6/01 |
|
|
|
|
|
taken |
on 4&9 |
|
|
|
|
|
|
|
|
||||||||||||||
|
Morning |
|
|
2 |
|
|
|
|
|
|
|
|
3 |
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
|
|
|
1,3,4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
bleached |
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
|
|
|
2 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Thursday |
|
|
1 |
|
|
photographs taken |
|
|
1,6,7,9 |
|
|
|
|
|
|||||||||||||||
|
12/13/01 |
|
|
|
|
|
with digital camera |
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Morning |
|
|
2 |
|
|
|
|
|
3 |
|
|
5 |
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
5 |
|
|
4 |
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
|
|
|
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Thursday |
|
|
1 |
|
|
|
|
|
1,6,7,9 |
2,3,4,5,8,10,11 |
|
|
|
|
|
||||||||||||||
|
12/20/01 |
|
|
2 |
|
|
|
|
|
3,4 |
1,2,6,7 |
|
5 (bleached) |
|
|
|||||||||||||||
|
Morning |
|
|
|
|
|
|
|
|
|
|
|
8 (covered with algae) |
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
5 |
2,6,9 (porites) |
1,3 (broken) |
4 (bleached) |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
7,8,10,11 (leaf) |
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
1,2 |
3,4,5,6,7,8, |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
9,10,11,12,13 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Wednesday |
|
|
1 |
|
|
|
|
|
1,6,7,9 |
2,3,4,5,11 |
8 (bleached tips) |
|
|
|
|
||||||||||||||
|
12/26/01 |
|
|
|
|
|
|
|
|
|
|
10 (becoming |
|
|
|
|
||||||||||||||
|
Morning |
|
|
|
|
|
|
|
|
|
|
covered with algae) |
|
|
|
|
||||||||||||||
|
|
|
|
2 |
|
|
|
|
|
3,4,8 |
1,2,6,7 |
|
5 |
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
5 |
2,6,9 (porites) |
1,3 |
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
7,8,10,11 (leaf) |
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
1,2 |
3,4,5,6,7,8,9 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
10,11,12,13 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Wednesday |
|
|
1 |
|
|
Some corals broken |
|
|
1,6,7,9 |
2,3,4,5,11 |
8,10 (covered with algae) |
|
|
|
|
||||||||||||||
|
1/2/02 |
|
|
2 |
|
|
possibly by divers. |
|
|
3,4,8 |
1,2,6,7 |
|
5 |
|
|
|
||||||||||||||
|
Morning |
|
|
3 |
|
|
Asked dive staff |
|
|
5 |
2,6,7,8,9,10,11 |
1,3 |
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
to warn divers about |
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
the balls |
|
|
1,2 |
3,4,5,6,7,8,9, |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
10,11,12,13 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Friday |
Windy |
|
1 |
|
|
1 large acropora |
Lots of Acropora |
|
16,7,9 |
4 |
3 (acropora bleaching) |
2,5,11 (acropora |
Octopus and pincusion |
|
|
||||||||||||||
|
1/11/02 |
Sea choppy |
|
|
|
|
(attaced with putty) |
Bleaching |
|
|
|
8,10 becoming covered |
bleached and dead.) |
sea star inside reef balls. |
|
|
||||||||||||||
|
Morning |
|
|
|
|
|
bleached and dead. |
|
|
|
|
with algae |
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
The other is bleaching |
|
|
|
|
|
|
Eggs laid inside RB 3 - |
|
|
||||||||||||||
|
|
|
|
2 |
|
|
|
|
|
|
|
|
|
possibly octopus but more |
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
3,4,8 |
1,2,6,7 |
|
5 |
likely a shell. |
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
5 |
1,2,3,6,7,8,9,10,11 |
|
4 |
Lots of surgeon fish feeding |
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
on algae. Also many pipe |
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
fish and gobies on balls, and |
|
|
||||||||||||||
|
|
|
|
|
|
|
|
Leaf corals appear to |
|
1,2 |
5,6,7,8,9,10,11,12,13 |
3+4 bleaching |
|
some wrasse displaying |
|
|
||||||||||||||
|
|
|
|
|
|
|
|
have some blackening |
|
|
|
|
|
terratorial behaviour. |
|
|
||||||||||||||
|
|
|
|
|
|
|
|
of tissues. |
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
Will continue to monitor |
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Wednesday |
Calm |
|
1 |
|
|
2 large acroporas now |
|
|
1,6,7,9 |
3,4 |
8&10 covered with algae |
2,5,11 (acropora |
|
|
|
||||||||||||||
|
1/16/02 |
Sunny |
|
|
|
|
completely bleached |
|
|
|
|
|
bleached and dead.) |
|
|
|
||||||||||||||
|
Morning |
|
|
2 |
|
|
|
|
|
|
6,7 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
Have noticed some |
|
|
3,4,8 |
1,2 (acropora) |
|
5 |
banded coral shrimp |
|
|
||||||||||||||
|
|
|
|
3 |
|
|
small-scale bleaching |
|
|
5 |
3,7,8,10,11 |
2 (porites) |
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
of acropora on the |
|
|
|
1,6,9 (porites) |
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
reef close to RB1 |
|
|
1,2 |
5,6 -13 |
3&4 (porites-some bleaching) |
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Wednesday |
Calm |
|
1 |
|
|
|
|
|
1,6,9,7 |
3,4 |
8&10 covered with algae |
2,5,11 bleached and dead |
|
|
|
||||||||||||||
|
1/23/02 |
Good Visability |
|
2 |
|
|
Some filamentous |
|
|
3,4,8 |
1&7 (bleached tips) |
|
5 |
|
|
|
||||||||||||||
|
Morning |
|
|
|
|
|
algae growing near |
|
|
|
2,6 (some blackening |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
base |
|
|
|
of tissue) |
|
|
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
5 |
1,3,6,7,8,9,10,11 |
2 (very small & nearly dead) |
4 bleached and dead |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
Some blackening of |
|
1,2 |
3,4,5,6,7,8,9,10,11 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
tissues of leaf coral |
|
|
12,13 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Wednesday |
Medium |
|
1 |
|
|
|
Algae and some ascidians |
|
1,6,9,7 |
3,4 |
8,10 |
2,5,11 |
|
|
|
||||||||||||||
|
1/30/02 |
Current |
|
|
|
|
|
now covering bleached |
|
|
|
|
|
|
|
|
||||||||||||||
|
Morning |
|
|
|
|
|
|
Acroporas |
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
2 |
|
|
|
Purple sponge |
|
3,4,8 |
1,2,6,7 |
|
5 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
growing close to |
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
Acropora - removed |
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
Some blackening of |
|
5 |
1,3,6,7,8,9,10,11 |
2 |
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
tissues of leaf coral |
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
Blackening of tissues of |
|
|
|
11 - almost completely gone |
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
leaf coral more pronounced |
|
1,2 |
3,4,5,6,7,8,9,10,12,13 |
only base remains - (eaten?) |
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
6 - edges look like they may |
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
have been eaten |
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Wednesday |
Calm |
|
1 |
|
|
|
|
|
1,6,7,9 |
3 (small) |
8 and 10 (nearly dead) |
2,5,11 |
|
|
|
||||||||||||||
|
2/6/02 |
Good |
|
|
|
|
|
|
|
|
4 (small) |
|
|
|
|
|
||||||||||||||
|
Morning |
Visability |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
2 |
|
|
|
|
|
3,4,8 |
1,2,6 (small),7 |
|
5 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
Attached Acropora to |
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
Plug 4 with putty |
|
5 |
1,2,3,6,7,8,9,10,11 |
|
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
10 (leaf) broken off - |
|
1,2,10,11 |
3 (small), 4(broken) |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
reattached with putty |
|
|
5,6,7,8,9,13,12 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Wednesday |
|
|
1 |
|
|
|
|
|
1,6,7,9 |
3 (small), 4 |
8 and 10 (covered with algae) |
2,5,11 |
Lionfish inside RB |
|
|
||||||||||||||
|
2/13/02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Morning |
|
|
2 |
|
|
|
|
|
3,4,8 |
1,2,6,7 |
|
5 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
5 |
1,2, 3(v. small),6,7,8 |
|
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
9,10,11 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
10 (leaf) broken off plug |
|
1,2,10,11 |
3,4,5,6,7,8,9,13,12 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Wednesday |
|
|
1 |
|
|
|
|
|
1,6,7,9 |
3,4 |
8,10 |
2,5,11 |
|
|
|
||||||||||||||
|
2/27/02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Morning |
|
|
2 |
|
|
|
Attached porites to 3&4 |
|
3,4,8 |
1,2,6,7 |
|
5 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
5 |
1,2,3,6,7,8,9,10,11 |
|
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
1,2,10,11 |
3,4,5,6,7,8,9,13,12 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Wednesday |
|
|
1 |
|
|
|
|
|
1,6,7,9 |
3,4 |
8,10 |
2,5,11 |
|
|
|
||||||||||||||
|
3/6/02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Morning |
|
|
2 |
|
|
|
|
|
3,4,8 |
1,2,6,7 |
|
5 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
5 |
1,2,3,6,7,8,9,10,11 |
|
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
1,2,10,11 |
3,4,5,6,7,8,9,12,13 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Friday |
|
|
1 |
|
|
|
|
|
1,6,7,9 |
3,4 |
8,10 |
2,5,11 |
|
|
|
||||||||||||||
|
3/29/02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Morning |
|
|
2 |
|
|
|
|
|
3,4,8 |
1,2,6,7 |
|
5 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
5 |
1,2,3,6,7,8,9,10,11 |
|
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
1,2,10,11 |
3,4,5,6,7,8,9,12,13 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Saturday |
|
|
1 |
|
|
|
|
|
1,6,7,9 |
3,4 |
|
2,5,8,10,11 |
|
|
|
||||||||||||||
|
4/6/02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Morning |
|
|
2 |
|
|
|
|
|
3,4,8 |
1,2,6,7 |
|
5 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
5 |
1,2,3,6,7,8,9,10,11 |
|
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
1,2,10,11 |
3,4,5,6,7,8,9,12,13 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Saturday |
|
|
1 |
|
|
|
|
|
1,6,7,9 |
3,4 |
|
2,5,8,10,11 |
|
|
|
||||||||||||||
|
4/14/02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Morning |
|
|
2 |
|
|
|
|
|
3,4,8 |
1,2,6,7 |
|
5 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
5 |
1,2,3,6,7,8,9,10,11 |
|
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
1,2,10,11 |
3,4,5,6,7,8,9,12,13 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Wednesday |
|
|
1 |
|
|
|
|
|
1,6,7,9 |
4 |
|
2,3,5,8,10,11 |
|
|
|
||||||||||||||
|
4/17/02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Morning |
|
|
2 |
|
|
Re-assessed plug numbers & |
|
|
3,4,8 |
1,2,6,7 |
|
5 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
species to produce map of |
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
plug locations |
|
|
5 |
1,2,3,6,7,8,9,10,11 |
|
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
1,2,10,11 |
3,4,5,6,7,8,9,12,13 |
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Friday |
|
|
1 |
|
|
|
|
|
1,6,7,9 |
4 |
|
2,3,5,8,10,11 |
Many ascidians and some |
|
|
||||||||||||||
|
4/24/02 |
|
|
|
|
|
|
|
|
|
|
|
|
filamentous algae growing on RB. |
|
|
||||||||||||||
|
Morning |
|
|
|
|
|
|
|
|
|
|
|
|
Lion fish & tiger cowrie inside RB. |
|
|
||||||||||||||
|
|
|
|
2 |
|
|
A lot of filamentous algae & |
|
|
3,4,8 |
1,2,6,7 |
|
5 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
ascidians growing on RB - |
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
removed. |
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
5 |
1,2,3,6,7,8,9,10,11 |
|
4 |
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
1,2,10,11 |
3,4,5,6 (broken),7,8,9, |
|
|
Wrasse displaying terratorial behaviour. |
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
12,13 |
|
|
Surgeon fish feeding on algae growing |
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
on RB |
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
|
Saturday |
|
|
1 |
|
|
|
|
|
1,6,7,9 |
4 |
|
2,3,5,8,10,11 |
|
|
|||||||||||||||
|
5/4/02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Morning |
|
|
2 |
|
|
Removed encrusting sponge |
|
|
3,4,8 |
1,2,6,7 |
|
5 |
|
|
|||||||||||||||
|
|
|
|
|
|
|
growing close to corals |
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
3 |
|
|
|
|
|
5 |
1,2,3,6,4,8,9,10,11 |
|
4 |
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
1,2,10,11 |
3,4,5,6,7,8,9,12,13 |
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Wednesday |
Sea very choppy |
|
1 |
|
|
|
|
|
1,6,7,9 |
4 |
|
2,3,5,8,10,11 |
|
|
|||||||||||||||
|
5/15/02 |
strong wind from |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Morning |
south-west |
|
|
|
|
|
Some sediment build up |
|
3,4,8 |
2,6 |
1 (almost completely bleached) |
5 |
|
|
|
|
|||||||||||||
|
|
|
|
2 |
|
|
|
on bleahced corals. |
|
|
|
7 (completely bleached and |
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
Possibly due to bad weather. |
|
|
|
probably dead) |
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
Some sediment build up |
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
3 |
|
|
|
on bleached coral. |
|
5 |
1,2,6,7,8,9,10,11 |
3 (completely bleached and |
4 |
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
Possibly due to bad weather. |
|
|
|
probably dead) |
|
|
|
|||||||||||||||
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
1,2,10,11 |
3,4,5,6,7,8,9,12,13 |
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
Most plugs empty or dead |
|
|
|
|
|
|
|
|||||||||||||||
|
Wednesday |
Sea medium |
|
1 |
|
|
lots of sand sediment |
ascidians growth all over, even |
|
all |
1 acropora on putty |
|
probably all |
grouper |
|
|||||||||||||||
|
12.6.02 |
bad visibility |
|
|
|
|
all over the rb |
on plugs. Removed the one |
|
|
|
|
|
|
|
|||||||||||||||
|
Morning |
|
|
|
|
|
|
on plugs |
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
2 |
|
|
lot of sand sediment |
Most plugs empty |
|
almost all |
1 acropora |
|
|
giant moray |
|
|||||||||||||||
|
|
|
|
|
|
|
covering rb |
ascidian growth all over |
|
|
1 dendrophillydae |
|
|
|
|
|||||||||||||||
|
|
|
|
|
|
|
lot of sediment |
|
|
|
1 porites |
|
|
|
|
|||||||||||||||
|
|
|
|
3 |
|
|
covering rb |
|
|
|
|
|
|
new acropora fragment with putty |
|
|||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
4 |
|
|
lot of sediment |
|
|
1,2,10,11 |
3,4 small porites |
|
|
new acropora fragment with putty |
|
|||||||||||||||
|
|
|
|
|
|
|
covering rb |
|
|
|
all rest ok |
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
only one plug left with |
|
1 |
1 acropora colony Ok |
|
all except 1 |
big porcupine fish indide |
|
|||||||||||||||||||||
|
Friday |
Sea calm |
|
1 |
|
Still sediment |
coral |
|
||||||||||||||||||||||
|
5.7.02 |
bad visibility |
|
taken pictures with Yoshie |
|
|||||||||||||||||||||||||
|
Morning |
|
|
|
|
|
digital camera |
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
2 |
|
|||||||||||||||||||||||||||
|
|
one plug left with coral |
|
all except three plugs |
1 acropora |
|
||||||||||||||||||||||||
|
|
1 demdrophylllidae |
|
almost all except 2 plugs |
acropora on putty ok |
|
||||||||||||||||||||||||
|
|
1 acropora colony ok |
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
3 |
|
|||||||||||||||||||||||||||
|
|
1,2,3, plugs porites ok |
|
1,2,3 porites plugs |
|
|||||||||||||||||||||||||
|
|
4,5,6,7 plugs dendro ok |
|
4,5,6,7 dendro plugs |
|
new acropora on putty is not there anymore |
|
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||
|
|
4 |
|
most plugs ok |
|
1,2,3 porites very small |
|
|||||||||||||||||||||||
|
|
4,5,6,7,8 dendro ok |
|
new acropora on putty is not there anymore |
|
|||||||||||||||||||||||||
|
|
one giant moray eel inside |
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
Sediment |
|
|||||||||||||||||||||||||||
|
Thursday |
Sea rough |
|
1 |
|
Sand |
only one plug with coral |
|
1 |
|
all except one plug |
|
||||||||||||||||||
|
11.7.02 |
Medium cyrrent |
|
many ascidians |
one coral without plug |
|
||||||||||||||||||||||||
|
Morning |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
2 |
|
2 plugs with coral |
|
plug 8 empty |
2 |
plug 2 probably dying |
5 plugs dead |
hawkfish inside branching coral |
|
|||||||||||||||||||
|
|
1 coral without plug |
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
3 |
|
7 plugs with coral |
|
7 |
|
3 |
|
|||||||||||||||||||||
|
|
1 small coral without plug |
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
4 |
|
9 plugs with coral |
|
2 plugs empty |
7 + 2 very small |
|
||||||||||||||||||||||
|
|
2 on the top are very small |
|
2 new coral on top plugs with glue |
|
|||||||||||||||||||||||||
|
|
2 new coral on top plugs with glue |
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
1 |
|
only one plug with coral |
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Monday |
sea rough |
|
sedi,ment |
|
|||||||||||||||||||||||||
|
15.7.02 |
medium current |
|
2 |
|
sand |
2 plugs alive |
|
2 plugs empty |
2 |
1 |
all dead except 2 |
|
|||||||||||||||||
|
morning |
visibility ok |
|
1 plug dying |
|
|||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|
|
|||||||||||||||||||||||||||
|
|
3 |
|
7 alive |
|
2 empty |
7 |
1 |
1 |
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
4 |
|
9 |
|
1 |
2 new corals on plugs |
|
||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
Monday |
sea calm |
|
1 |
|
only 1 plug alive |
|
1 new coral on plug |
|
|||||||||||||||||||||
|
22.07.02 |
no current |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
morning |
good visibility |
|
|||||||||||||||||||||||||||
|
|
2 |
|
1 alive |
|
2 empty |
1 |
2 |
5 |
1 new coral on plug |
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
3 |
|
7 |
|
2 |
7 |
1 |
1 |
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
4 |
|
11 |
|
1 |
11 |
|
1 |
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
Monday |
|
1 |
|
1 alive |
|
8 empty |
1 |
|
4 |
new coral dead |
|
||||||||||||||||||
|
29.7.02 |
sea very rough |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
Morning |
medium current |
|
|||||||||||||||||||||||||||
|
|
bad visibility |
|
2 |
|
1 alive |
|
2 empty |
1 |
1 |
6 |
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
3 |
|
7 alive |
|
2 empty |
7 |
1 maybe |
1 |
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
4 |
|
10 alive |
|
3 |
10 |
|
1 |
new coral on plug dead - we removed it |
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
Monday |
sea rough |
|
1 |
|
1 alive |
|
8 empty |
1 |
|
3 |
|
||||||||||||||||||
|
5:08:02 |
medium current |
|
|||||||||||||||||||||||||||
|
Morning |
bad visibility |
|
2 |
|
1 alive |
|
2 empty |
1 |
1 |
6 |
|
||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
3 |
|
7 alive |
|
2 empty |
7 |
|
2 |
|
||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
4 |
|
10 alive |
|
3 empty |
10 |
|
damsel fish defending algae area |
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
Monday |
sea calm |
|
1 |
|
1 alive |
|
8 empty |
1 |
|
4 |
|
||||||||||||||||||
|
12:08:02 |
no current |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
good visibility |
|
|||||||||||||||||||||||||||
|
|
2 |
|
1 alive |
|
2 empty |
1 |
|
7 |
porcupine fish |
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
3 |
|
7 alive |
|
2 empty |
7 |
|
2 |
|
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
|
|
|||||||||||||||||||||||||||||
|
|
4 |
|
9 alive |
|
3 empty |
9 |
|
1 |
giant moray-damsel fish |
|
|||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
|
|
|||||||||||||||||||||||||||||

